Which of the following is the atomic number of an atom that has 12 protons and 12 neutrons?
A. 24
B. 12
C. 1
D. 144
The atomic number of an atom is equal to the number of protons in its nucleus.
In this case, the atom has 12 protons, so its atomic number is 12.
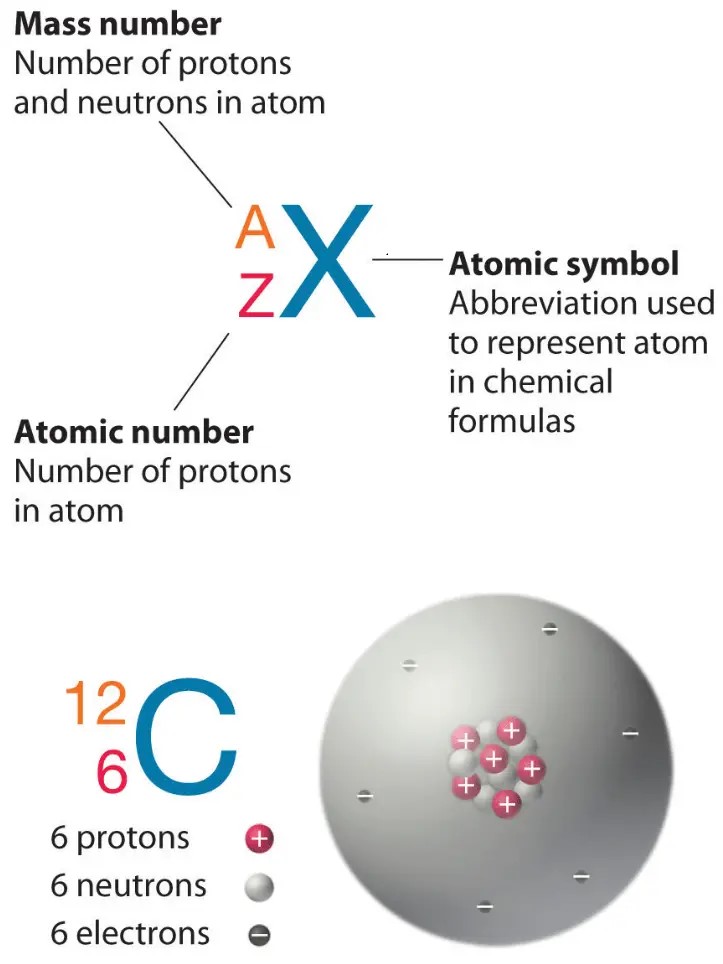
Choice A, 24, is not the correct answer because it represents the sum of the number of protons and neutrons in the atom’s nucleus, which is known as the mass number.
Choice C, 1, is not the correct answer because it does not represent the number of protons in the atom’s nucleus.
Choice D, 144, is not the correct answer because it represents the square of the mass number and does not represent any property of the atom.
Therefore, the Correct Answer is B.