Which of the following ions binds to the troponin complex, initiating contraction of a muscle?
A. Potassium.
B. Calcium.
C. Phosphorus.
D. Sodium
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape.
This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes crossbridge formation, and contraction of the muscle begins.
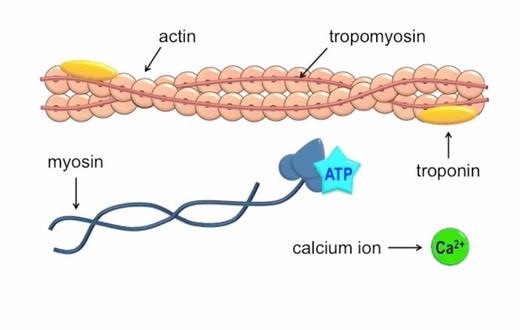
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism, but they do not bind to the troponin complex.
Sodium ions are important for generating action potentials, but they do not bind to the troponin complex.
Therefore, the Correct Answer is B.