Which of the following glands synthesizes antidiuretic hormone?
A. Pineal gland
B. Thymus
C. Hypothalamus
D. Pancreas
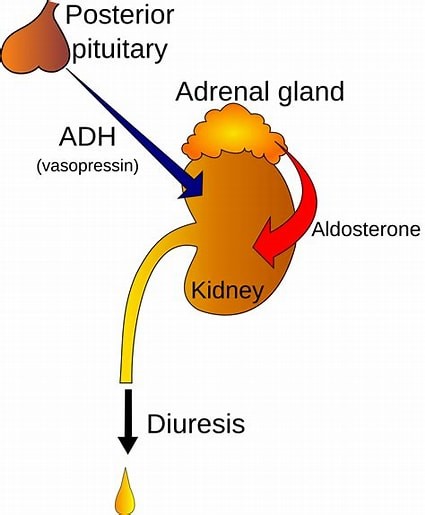
The hypothalamus is a region of the brain that synthesizes antidiuretic hormone (ADH), also known as vasopressin.
ADH is then transported to the posterior pituitary gland via neurohypophysial capillaries, where it is stored until it is ready to be secreted into the circulation.
Choice A.
Pineal gland is not correct because it is a small endocrine gland located in the brain that secretes the hormone melatonin, which regulates sleep-wake cycles, but it does not synthesize ADH.
Choice B.
Thymus is not correct because it is a gland located in the chest that produces hormones involved in immune system development, but it does not synthesize ADH.
Choice D.
Pancreas is not correct because it is a gland located behind the stomach that secretes hormones such as insulin and glucagon, which regulate blood sugar levels, but it does not synthesize ADH.
Therefore, the Correct Answer is C.