Which of the following determines the strength of an acidic solution?
A. Litmus paper that turns red
B. Litmus paper that turns blue
C. Measured pH value equal to 7
D. Measured pH value less than 7
Both litmus paper and a pH scale can be used to indicate whether a solution is acidic. However, a pH scale can also determine the strength of an acid.
Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
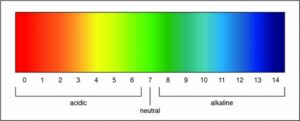
Therefore, the Correct Answer is D.


