Answer Explanation
Unlike condensation, deposition, and melting, evaporation is dependent not only on the temperature, but also on the amount of a substance available.
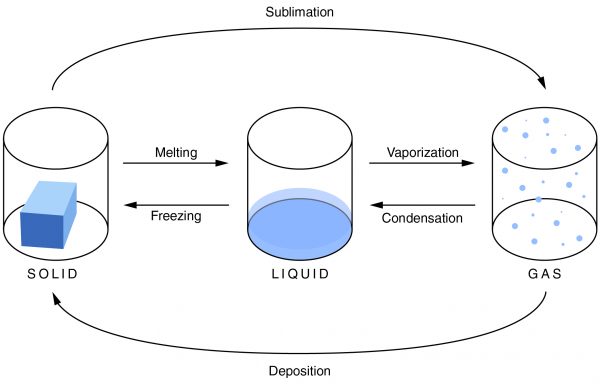
Condensation is the change of a gas or vapor to a liquid. A change in the pressure and the temperature of a substance causes this change. The condensation point is the same as the boiling point of a substance. It is most noticeable when there is a large temperature difference between an object and the atmosphere. Condensation is also the opposite of evaporation.
Evaporation is the change of a liquid to a gas on the surface of a substance. This is not to be confused with boiling, which is a phase transition of an entire substance from a liquid to a gas. The evaporation point is the same as the freezing point of a substance. As the temperature increases, the rate of evaporation also increases. Evaporation depends not only on the temperature, but also on the amount of substance available.
Freezing is the change of a liquid to a solid. It occurs when the temperature drops below the freezing point. The amount of heat that has been removed from the substance allows the particles of the substance to draw closer together, and the material changes from a liquid to a solid. It is the opposite of melting.
Melting is the change of a solid into a liquid. For melting to occur, enough heat must be added to the substance. When this is done, the molecules move around more, and the particles are unable to hold together as tightly as they can in a solid. They break apart, and the solid becomes a liquid.
Sublimation is a solid changing into a gas. As a material sublimates, it does not pass through the liquid state. An example of sublimation is carbon dioxide, a gas, changing into dry ice, a solid. It is the reverse of deposition.
Deposition is a gas changing into a solid without going through the liquid phase. It is an uncommon phase change. An example is when it is extremely cold outside and the cold air comes in contact with a window. Ice will form on the window without going through the liquid state.



