What is the role of calcium in muscle contraction?
A. Calcium binds to tropomyosin to expose the myosin-binding sites on actin.
B. Calcium is released from the sarcoplasmic reticulum to initiate the sliding of actin and myosin filaments.
C. Calcium activates the motor neurons to stimulate muscle contraction.
D. Calcium is required for the relaxation of muscles after contraction.
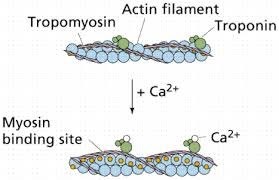
Muscle contraction is a complex process that involves the interaction between actin and myosin filaments in the muscle fibers. The sliding of these filaments is initiated by the release of calcium ions from the sarcoplasmic reticulum, a specialized organelle in muscle cells. The calcium ions bind to the protein troponin, which causes a conformational change in the troponin-tropomyosin complex, exposing the myosin-binding sites on actin. This allows the myosin heads to bind to actin, forming cross-bridges that pull the actin filaments towards the center of the sarcomere, resulting in muscle contraction.
Option a) is incorrect because calcium does not bind to tropomyosin directly, but rather binds to the protein troponin, causing a conformational change in the troponin-tropomyosin complex. Option c) is incorrect because calcium does not activate motor neurons, but rather is released from the sarcoplasmic reticulum in response to an action potential that travels down the motor neuron to the neuromuscular junction. Option d) is incorrect because calcium is required for muscle contraction, not relaxation. The relaxation of muscles after contraction is due to the active transport of calcium ions back into the sarcoplasmic reticulum, which allows the troponin-tropomyosin complex to return to its resting conformation, blocking the myosin-binding sites on actin and ending the cross-bridge cycle.
Therefore, the Correct Answer is B.