In a phase diagram, which of the following term is used for a substance held at a temperature and pressure where solid, liquid, and gaseous states of a substance exist simultaneously?
A. Critical temperature
B. Triple point
C. Critical point
D. Absolute zero
phase diagrams are used to indicate physical states, a substance exist in specific conditions of pressure and temperature. Besides, phase diagrams provide information on pressure dependence of the phase-transition temperatures such as melting, sublimation, and boiling points. The figure below shows a typical phase diagram.
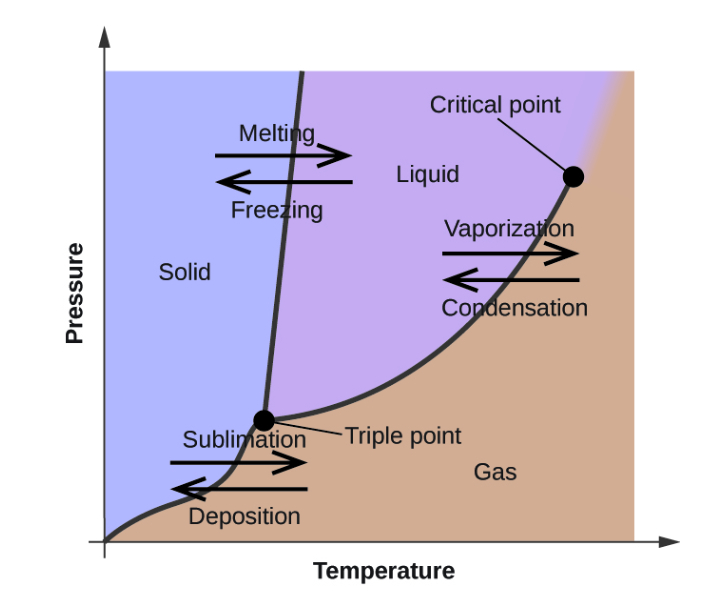
From the above diagram, the point where a substance exists in three states i.e solid, gas, and liquid is known as the triple point.
Therefore, the Correct Answer is B.


