For which of the following reasons does a chloride ion have a negative charge?
A. It gained an electron
B. It lost an electron.
C. It lost a proton.
D. It gained a proton.
A chloride ion has a negative charge because it gained an electron. When an atom gains an electron, it becomes negatively charged because it now has more electrons than protons. In the case of a chloride ion, the neutral chlorine atom gains an electron to become a negatively charged chloride ion.
The other options are incorrect because they do not result in a negative charge. Losing an electron would result in a positive charge. Losing or gaining a proton would change the identity of the atom and is not related to the formation of a chloride ion.
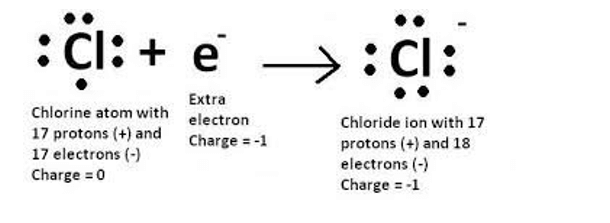
Therefore, the Correct Answer is A.